Implanting innovation
Leverage the latest innovations to reduce costs, advance quality and extend your production capacity.

Orthopedic Implant Manufacturing
Leverage our agility, ingenuity, automation and low-cost base to drive down your cost of manufacture.

Additive Manufacturing
The expertise, technologies, and scale to deliver end-to-end, world-class, additive manufacturing solutions.

Medical Component Manufacturing
Leverage precise, state-of-the-art Swiss turning technologies to manufacture high quality, complex components.
Capabilities
Joint Partnership
We help drive progress for our medical device customers by enhancing both their production capacity and their product and process innovation.
Manufacturing Capabilities
The facilities, technologies and processes to deliver end-to-end manufacturing.
Value-Add Capabilities
The team, expertise, and ingenuity to drive tangible added-value for our customers.
Devices
Extend capacity & reduce costs
Tap into our passion for innovation and we’ll help advance quality, extend your capacity and cut production costs.
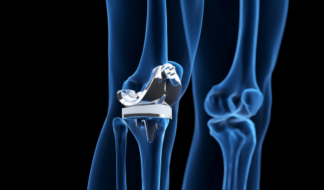
Knee Implants
Femoral implants, patella and tibia implants, and limb salvage.
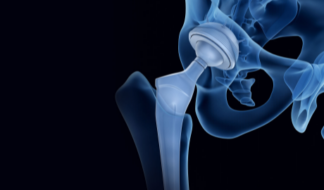
Hip Implants
Hip stems, acetabular cups, femoral heads and femoral liners.
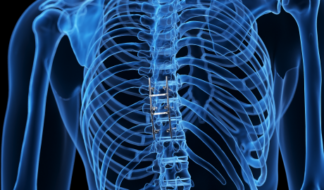
Spine Implants
Interbody cages, pedicle screws and rods, and SI fixation screws.
Trauma & Extremities
Shoulder & elbow, foot & ankle, tibia & femur, hand & wrist, cranial & maxofacial.


Additive Manufacturing: Understanding Process Validation Requirements for AM Orthopedic Products
Croom Medical has been a pioneer in the development of metallic additive manufacturing within the SME orthopedic sector since 2010. With Additive Manufacturing (AM) and ...

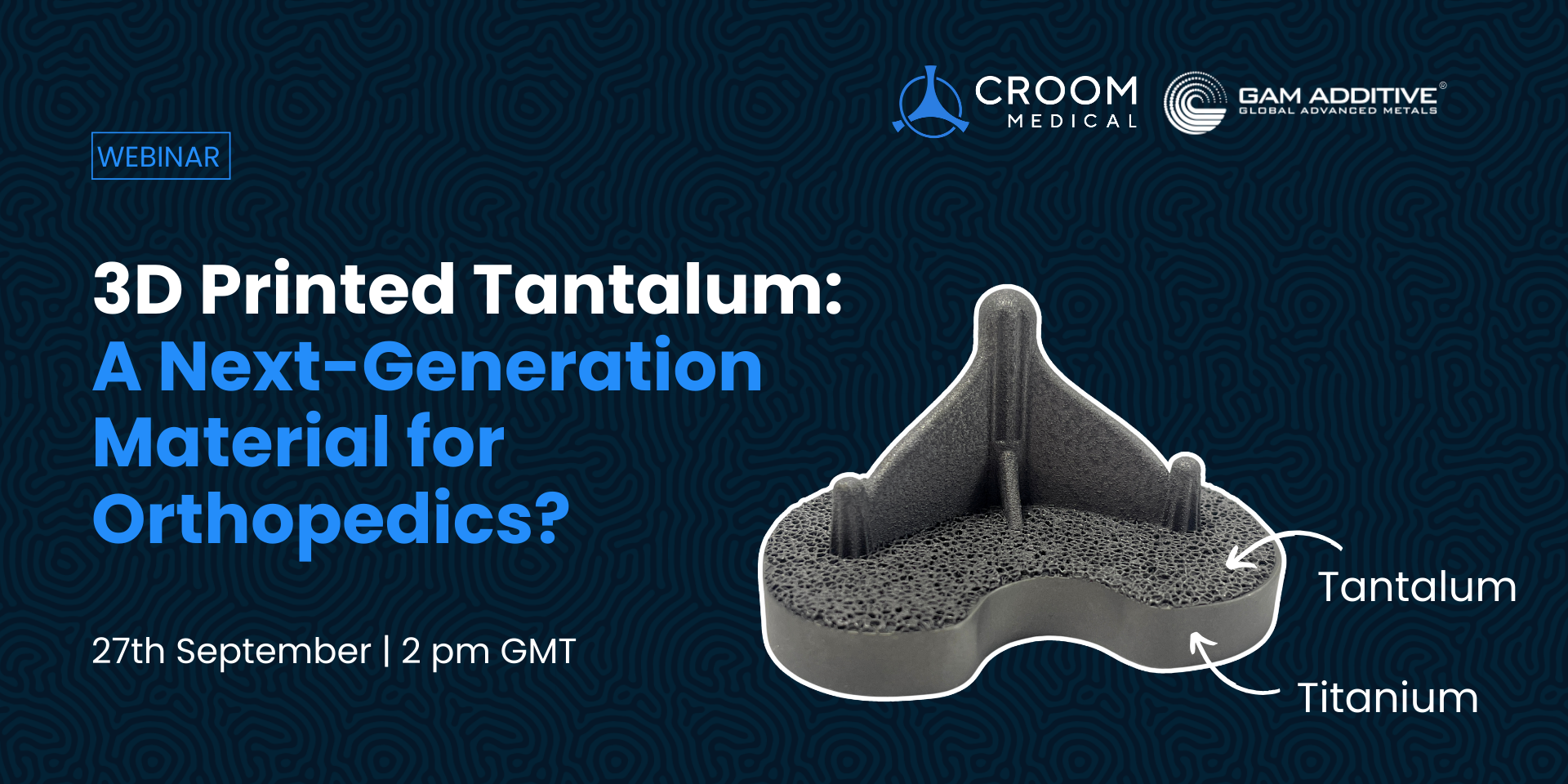
Croom Medical Partners with Global Advanced Metals to Present Breakthrough Tantalum Additive Manufacturing at OMTEC 2023
Limerick, Ireland – June 8, 2023 Croom Medical, a trusted leader in orthopaedic contract manufacturing, is pleased to collaborate with Global Advanced Metals (GAM) for ...